The Cinderella Molecule – Optimized Route to a Photolatent Base

There are many functional molecules of any kind: dyes, catalysts, inhibitors, initiators, auxiliaries, protecting and enabling compounds, stabilizers, preserving agents, enhancers, modifiers, sensitizers, coagulants, moisturizing and drying additives, indicators and many more…
All these functional molecules play an essential role in the final properties of a material, a consumer product or in a processing step. Nevertheless, they are unknown to most consumers, and their role is underestimated as they mainly act “in the background”, their quantity is low or their role simply is not that obvious to everyone who uses the product or the material, as well as who benefits from its outstanding performance.
One class of these “Cinderella molecules” are so called latent bases. What exactly does that mean? A base is a compound that provides a pH above 7 – but what means “latent”?
A “latent base” essentially is a kind of a protected base that in its protected state does not affect the pH. However, as soon as the protecting group is removed, it becomes a real base. The removal of the protective group is triggered by a specific stimulant.
What does one need such a compound for? The answer is quite obvious: Whenever you wish to trigger a reaction that is initiated or accelerated by a strong base AND you wish to trigger this reaction not now but at some time in the future long after having added the potential base to your mixture of starting materials.
Hence, such a latent base enables the preparation of reaction mixtures that only react when the protecting group is removed at any clearly defined point in time when desired. This, comprehensively, is quite an important feature of process control in converting such a raw material mixture to the final object. The end user doesn’t have to handle strong bases and to care about mixing it with other agents at a given stoichiometry but they simply have to remove the protecting group and, hence, unleash the power of this so far hidden base.
Remains the question: How easily can the protecting group be removed? How to set the trigger?
With “photolatent bases”, the name already indicates the approach to this problem: One simply has to apply light to the mix of starting materials. The energy of this radiation breaks the covalent bonds between a basic functional group (mostly amines) and the hitherto protecting moiety. The basic power gets liberated, the pH rises and the so far hindered reaction gets initiated. One such typical reaction can be a polymerization yielding a new (solid) material. Using light as stimulant for the deprotection reaction -an alternative stimulant, for example, is heat- provides the advantage of high spatiotemporal control, meaning that besides a well-defined point in time, defined by the switching-on of the light, the deprotection reaction occurs in well-defined areas in space, only in the regions of the formulation subjected to the light. This allows for an image-wise control of the polymerisation process and thus in the possibility of producing structures.
Bearing such polymerisation options in mind, possible applications become more obvious:
One can use mixtures of monomers and photolatent bases with products in the fields of dental inks in the printing industry, coatings for the automotive industry, or for protective coatings on wood (e.g. furniture), or on printed paper, for “self-healing” surfaces and for many applications in the promising 3D-printing industry. The option to use light as a trigger, makes these photolatent bases even more valuable: As the light beam can be focused and regulated regarding intensity, wavelength and direction, such reactions proceed in a clearly defined way at a clearly set location at a given time. These features pave the way for selective polymerisations resulting in structured materials.
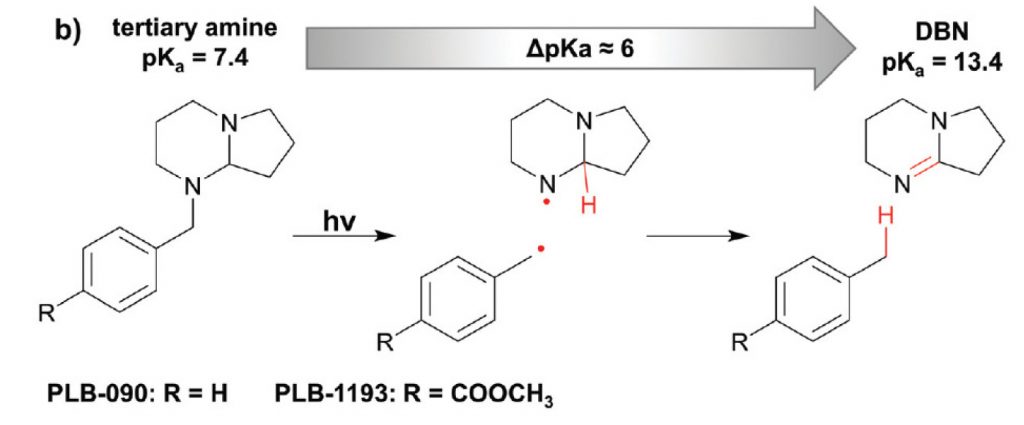
The general principle of the way of action of such a photolabile base is depicted in the following scheme[1]:
[1] K. Dietliker et. al.; Polym. Chem., 2022, 13, 1169–1176
The strongly basic DBN is protected by a benzyl group that proved to be photo-labile. The energy of light removes the benzyl moiety, giving rise to a pKa change of ~6 units! Some practical applications of this photolatent base and special conditions of its activation and use are described in the same paper.
Despite these attractive properties, there is one problem for the wide industrial application of this type of reactions: These protected bases (e.g., SCD-PLB-090 shown in the figure above) are not commercially available on larger scales. At least, they have not been available until recently. While there is a huge demand in many industries, the hitherto used synthesis of SCD-PLB-090 simply was too tedious, hence, too expensive.
This discrepancy of market demand on the one hand and lacking availability on the other hand initiated a co-operation between the companies SCD Dr. Sommerlade Chemistry Design GmbH and ChiroBlock GmbH about two years ago.
Dr. Sommerlade and his colleagues have gained much experiences with both the process development for this latent base and with the special needs of potential industrial customers. They also designed a new, more efficient synthetic protocol, which will result in a significantly cheaper product. However, they did not have access to larger scale synthetic facilities, or to a lab that is used to deal with new synthetic approaches, which by optimization of reactions result in a robust and scalable production process at reasonable costs.
These requirements fit well to the competences of ChiroBlock. With its more than 20 years’ experience in the field of applied synthetic development and h its talented team of chemists, ChiroBlock proved to be the ideal complementary partner to SCD.
During the last two years, both parties had been jointly developing h a new synthesis protocol before it finally was successfully scaled up and validated early in 2022. Already on a lower kg-scale, the overall costs of the product could be more than halved compared to the initial research-based protocol. Expensive operations such as hydrogenation or distillation could be replaced by cheaper ones. Toxic solvents and reagents were replaced by safer chemicals. Last but not least, the multi-step process with the need of work-up and purification of intermediates could be streamlined to a two-step protocol yielding PLB-090 in good yields and quality with no extra purification operation required.
These positive results enable the partners to now offer this valuable compound to interested customers at a competitive price. As this synthesis is easily scalable, we prospectively can meet any demand in volumes. This is good news not only for existing customers but also for the general R&D scene and industry in the field of high-performance polymers.
Additionally, this outcome proves the efficiency and quality of the bilaterally open collaboration between our two companies; it is a good example for the ability of such collaborations in partnership to solve both technical and marked oriented problems by combining forces, ideas, resources and expertise of SCD and ChiroBlock.